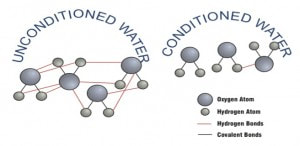
The water molecules link up to each other because of the diple nature of each individual water molecule. Oxygen is more electro-negative (attracts electrons) than hydrogen; as a result, the electrons will spend a greater part of their time orbiting the oxygen atom versus the hydrogen atom. This creates the dipole. The additional negative energy in the water, as a result of the E.S.P., reduces the bonding of the oxygen atoms of water molecules with the hydrogen atoms of adjacent molecules.
This means there are fewer bonds between each water molecule, thus making the water "wetter".